April 9, 2020 - Many questions are circulating concerning the number of COVID-19 positive cases as well as how those numbers are being generated and how the varying test results are impacting the Texas Department of State Health Services (TxDSHS) reported cases for Shelby County.
The confusion has developed due to public notification by some Shelby County citizens who received an in office positive test result and later received a negative test result via a different testing medium. Which test was correct and why didn't the reported positive cases for Shelby County decrease with the negative test result?
The number of positive cases of COVID-19 released by the TxDSHS is based only on testing results from molecular testing or swab testing; therefore, the in office positive test was not included in their numbers.
No testing is 100% accurate, and as more studies are released, a conclusion can be drawn that the number of positive cases of COVID-19 is being under reported.
Where are they going with those swabs?
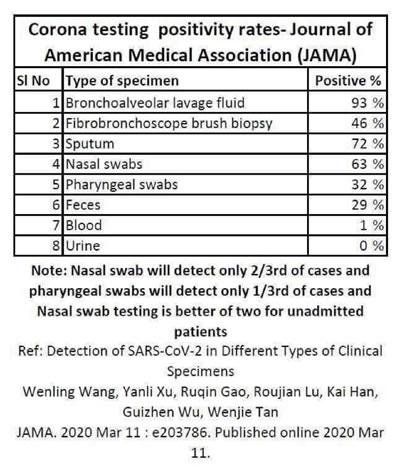
The Centers for Disease Control (CDC) chose to use the swab testing as the way of confirming positive cases early on amid limited options for detecting the virus. However, the accuracy of swab testing has been in question. A local doctor in Center submitted to Shelby County Today (SCT) the below chart showing 'Corona testing positivity rates - Journal of American Medical Association (JAMA)' which showed the positive percentage rates of different types of specimen. The Nasal swab (nasopharyngeal swab) showed a 63% positive rate out of known positive cases, which is only a two-third detection accuracy rate. The pharyngeal swab (a swab of the back of the throat) showed a 32% positive rate in the same case study which is only a one-third detection accuracy rate. (Source: Detection of SARS-CoV-2 in Different Types of Clinical Specimens)
At this time, there are two testing options in Shelby County: (1) molecular testing (swab tests) and now (2) serological testing (blood sample rapid test), which tests specifically for exposure to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19. The serological tests were approved for use under the Emergency Use Authorization (EUA) on March 16, 2020. (Accelerated Emergency Use Authorization (EUA) Summary, COVID-19 RT-PCR Test)
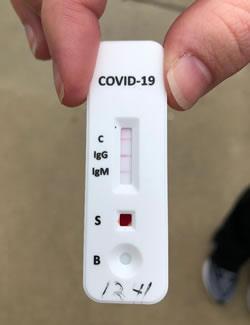 The test cassette has three indicators: C (Control), IgG, and IgM. The test is conducted by taking a small finger-prick blood sample. The presence of immunoglobulin M (IgM) antibodies indicate recent exposure to COVID-19, while the presence of immunoglobulin G (IgG) antibodies indicate later-stage infection.
The test cassette has three indicators: C (Control), IgG, and IgM. The test is conducted by taking a small finger-prick blood sample. The presence of immunoglobulin M (IgM) antibodies indicate recent exposure to COVID-19, while the presence of immunoglobulin G (IgG) antibodies indicate later-stage infection.
- C - Control for the test.
- IgM - Symptomatic 3-5 days, Asymptomatic 7 days. The test indicates the presence of IgM anti-COVID-19 in the specimen. The result is IgM anti-COVID-19 positive.
- IgG - The test indicates for the presence of IgG anti-COVID-19 in the specimen. The result is IgG anti-COVID-19 positive.
The rapid test is being administered by Aurora Concepts and it is the CoronaChek, COVID-19 IgG/IgM Rapid Test Cassette. The test can be used as an auxiliary means of detecting COVID-19. The product states an accuracy rate of 99.6% for the IgG anti-COVID-19 in the specimen and a rate of 97.8% accuracy for IgM. On the test strip, the C should always show positive to indicate a good test strip. Then, if the IgG and IgM do not indicate anti-COVID-19, then the results are negative. If the IgG and/or the IgM show positive, the test is positive. The IgG indicates a later-stage infection and the IgM indicates a more recent exposure and infection.
If a test returns a positive result, the patient is given a swab test that is sent off to a lab for processing. If the swab test returns a positive, the confirmed case is reported to the TxDSHS to update their statistics for positive cases plus adding to the total tests. If the swab test returns a negative, the results are counted by TxDSHS as only an addition to the total tests.
Disclaimers are included with all testing options, which is true for the rapid test as well. Aurora Concepts provided SCT with their disclaimer. which is required by the Federal Drug Administration (FDA) to be included with all testing: "This test has not been reviewed by the FDA. Negative results do not rule out SARS-CoV-2 infection, particularly in those who have been in contact with the virus. Follow-up testing with a molecular diagnostic should be considered to rule out infection in these individuals. Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection status. Positive results may be due to past or present infection with non-SARS-CoV-2 coronavirus strains, such as coronavirus HKU1, NL63, OC42, or 229E."
Disclaimers are what they are - a warning which can be applied to all testing options. None of the tests available are 100% although the rapid test data appears to be more accurate according to case studies. However, the only way for someone wanting to prevent catching COVID-19 is to treat everyone as if they are infected by staying away and to treat yourself as if you are infected by staying at home. People who are exposed to COVID-19 and are asymptomatic are a real concern to containing the spread of this deadly virus as they aren't aware they are carriers of the virus and are more likely not to be tested.
If you take the rapid test and receive a positive and then take the swab test and receive a negative, does that mean you don't have COVID-19 or that you can't get it? The only sure answer is, if you test positive for COVID-19 with either test and you are not needing hospitalization, please stay at home for two weeks at minimum as recommended by health officials. (The Science Behind A 14-Day Quarantine After Possible COVID-19 Exposure)
SCT has been notifed by Aurora Concepts and Hope Community Medicine that they provide COVID-19 testing.
- Aurora Concepts Offering Telehealth; COVID-19 Testing
- Hope Community Medicine Active Testing Site for COVID-19
Sources and more information since we all have more time to read:
- Testing Individuals for Coronavirus Disease 2019 (COVID-19)
- The COVID-19 Pandemic in the US: A Clinical Update
- COVID-19 IgG/IgM Rapid Test Cassette is specific for SARS-COV2
Clinical Evaluation Results: The clinical evaluation was carried out during February 2020 across 10 hospitals and reported on March 12, 2020. The results show that the testing reagent and reference reagent have equivalent effectiveness in detecting COVID-19 when tested in the same clinical specimens. Compared with the reference reagent, the positive agreement was 93.87% (95%CI:90.24%~96.46%), the negative agreement was 99.10% (95%CI:97.70%~99.75%) and total agreement was 97.19% (95%CI:95.65%~98.26%). The kappa value of the consistency analysis was 0.94 (95%CI:95.65%~98.26%). The results of the clinical evaluation show that the two reagents (methods) have a high degree of consistency and equivalent sensitivity and specificity in detecting COVID-19. - Evaluating the accuracy of different respiratory specimens in the laboratory diagnosis and monitoring the viral shedding of 2019-nCoV infections
- FAQs on Diagnostic Testing for SARS-CoV-2
- Coronavirus (COVID-19) Update: FDA Provides More Regulatory Relief During Outbreak, Continues to Help Expedite Availability of Diagnostics
- FDA Statement (Coronavirus (COVID-19) Update: Serological Tests)
- Coronavirus false test results: With the push to screen come questions of accuracy









